

Initial assessment
• Ultrasound should be used to exclude other causes of cholestasis.
• Physical examination should be used to screen for hepatomegaly, splenomegaly and extrahepatic signs
of advanced liver disease.
Unexplained cholestasis
• Elevated ALP but AMA-negative (<1/40)
• Concern over, or presence of, autoimmune hepatitis
↓
↓
If further assessments confirm PBC
*Liver biopsy is rarely required for diagnosis in the correct clinical context; consider if disease-specific autoantibodies are absent, or concern over features of AIH or coexistent NAFLD.
Disease management
Pruritus, fatigue, sicca complex, bone density, coexistent autoimmune disease, CV risk and metabolic syndrome
Assess pre-treatment disease stage and long-term risk of disease progression
• The strongest risk factors for inadequate response to UDCA are early age at diagnosis and advanced disease stage at presentation. Other risk factors also exist for inadequate response to UDCA, including male gender (associated with more advanced disease at presentation).
• Patients with an inadequate response to UDCA, and those with cirrhosis, are at the greatest risk of disease progression and complications from PBC.
• Severity of symptoms does not necessarily correlate with disease stage in PBC, however, severe pruritus can indicate an aggressively ductopenic variant of PBC, which is associated with a poor prognosis.
Hirschfield et al. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol
2017; 67(1): 145–172
Zhang et al. Early biochemical response to ursodeoxycholic acid and long-term prognosis of primary biliary cirrhosis:
results of a 14-year cohort study. Hepatology 2013; 58(1): 264–272 3. Corpechot et al. Noninvasive elastography-based assessment of liver
fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology 2012; 56(1): 198–208
Assess 1st line treatment response within 6–12 months
• Most biochemical response criteria have been validated as predicting long-term disease progression at 12 months from UDCA initiation.1 A 12-month period is conventionally used to assess biochemical response to UDCA, but it has been demonstrated that evaluation at 6 months may be equally discriminatory.
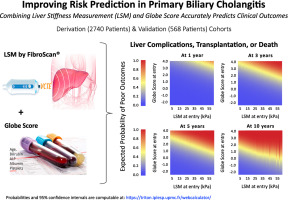
• Elastography should be used yearly to assess liver fibrosis. Elastography has been shown as one of the best surrogate markers for the detection of cirrhosis or severe fibrosis.1 It has also been demonstrated that liver stiffness measurements (as measured by elastography) of greater than 9.6kPa are associated with a 5-fold increased risk of liver decompensation, transplantation or death.3 However, the ability of elastography to predict disease progression in PBC requires further validation.
Hirschfield et al. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol
2017; 67(1): 145–172
Zhang et al. Early biochemical response to ursodeoxycholic acid and long-term prognosis of primary biliary cirrhosis:
results of a 14-year cohort study. Hepatology 2013; 58(1): 264–272 3. Corpechot et al. Noninvasive elastography-based assessment of liver
fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology 2012; 56(1): 198–208
Abbreviated Prescribing Information
Presentation: OCALIVA supplied as film-coated tablets containing 5 mg and 10 mg obeticholic acid.
Indication: For the treatment of primary biliary cholangitis (also known as primary biliary cirrhosis) in combination with ursodeoxycholic acid (UDCA) in adults with an inadequate response to UDCA or as monotherapy in adults unable to tolerate UDCA.
Dosage and administration: Oral administration. Hepatic status must be known before initiating treatment. In patients with normal or mildly impaired (Child Pugh Class A) hepatic function, the starting dose is 5 mg once daily. Based on an assessment of tolerability after 6 months, the dose should be increased to 10 mg once daily if adequate reduction of alkaline phosphatase (ALP) and/or total bilirubin have not been achieved. No dose adjustment of concomitant UDCA is required in patients receiving obeticholic acid. For cases of severe pruritus, dose management includes reduction, temporal interruption or discontinuation for persistent intolerable pruritus; use of bile acid binding agents or antihistamines (see SmPC).
Moderate to severe hepatic impairment: In patients with Child Pugh B or C hepatic impairment, a reduced starting dose of 5 mg once weekly is required. After 3 months, depending on response and tolerability, the starting dose may be titrated to 5 mg twice weekly and subsequently to 10 mg twice weekly (at least 3 days between doses) if adequate reductions in ALP and/or total bilirubin have not been achieved. No dose adjustment required in Child Pugh
Class A function.
Mild or moderate renal impairment: No dose adjustments are required. Paediatric population: No data. Elderly: No dose adjustment required; limited
data exist. Contraindications: Hypersensitivity to the active substance or any excipients. Complete biliary obstruction.
Special warnings and precautions for use: After initiation, patients should be monitored for progression of PBC with frequent clinical and laboratory assessment of
those at increased risk of hepatic decompensation. Dose frequency should be reduced in patients who progress from Child Pugh A to Child Pugh B or C Class disease.
Serious liver injury and death have been reported in patients with moderate/severe impairment who did not receive appropriate dose reduction. Liver-related adverse
events have been observed within the first month of treatment and have included elevations in alanine amino transferase (ALT), aspartate aminotransferase (AST) and
hepatic decompensation.
Interactions: Following co-administration of warfarin and obeticholic acid, International Normalised Ratio (INR) should be monitored and the dose of warfarin adjusted, if needed, to maintain the target INR range. Therapeutic monitoring of CYP1A2 substrates with narrow therapeutic index (e.g. theophylline and tizanidine) is recommended. Obeticholic acid should be taken at least 4-6 hours before or after taking a bile acid binding resin, or at as great an interval as possible.
Fertility, pregnancy and lactation: Avoid use in pregnancy. Either discontinue breast-feeding or discontinue/abstain from obeticholic acid therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman. No clinical data on fertility effects.
Undesirable effects: Very common (≥1/10) adverse reactions were pruritus, fatigue, and abdominal pain and discomfort. The most common adverse reaction
leading to discontinuation was pruritus. The majority of pruritus occurred within the first month of treatment and tended to resolve over time with continued dosing.
Other commonly (≥1/100 to <1/10) reported adverse reactions are, thyroid function abnormality, dizziness, palpitations, oropharyngeal pain, constipation, eczema,
rash, arthralgia, peripheral oedema, and pyrexia. Please refer to the SmPC for a full list of undesirable effects.
Overdose: Liver-related adverse reactions were reported with higher than recommended doses of obeticholic acid. Patients should be carefully observed, and supportive
care administered, as appropriate. Legal category: POM Marketing authorisation numbers: EU/1/16/1139/001 & 002
Marketing authorisation holder: Intercept Pharma Ltd, 2 Pancras Square, London, N1C 4AG, United Kingdom Package quantities and basic NHS cost: OCALIVA 5 mg and 10 mg £2,384.04 per bottle of 30 tablets. Date of revision: 11th April 2018